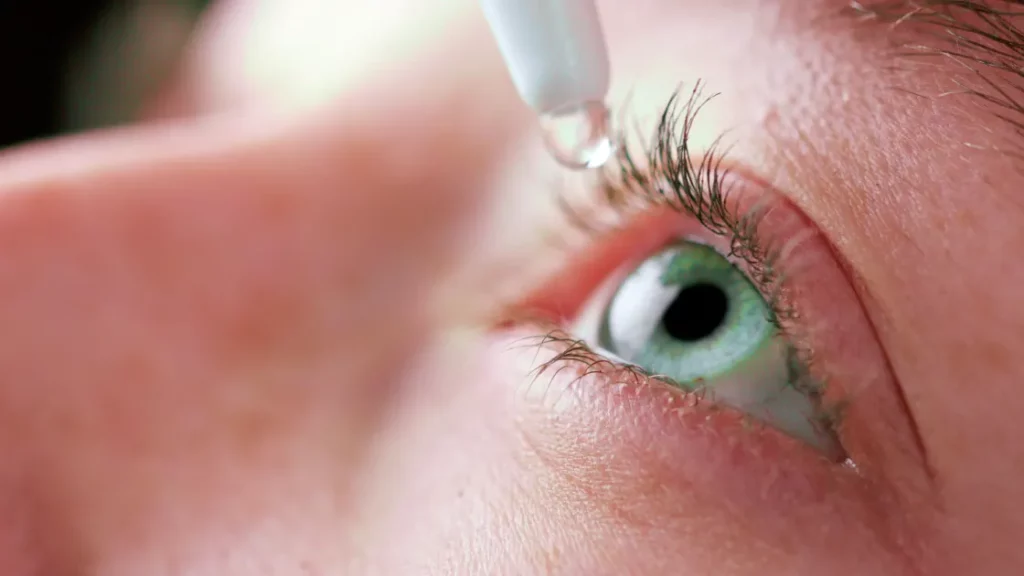
This is the third warning about eyedrops that the Food and Drug Administration has issued this year, and it has caused consumers to wonder which of the 27 types of eyedrops they should avoid using.
The FDA announced on Wednesday that Cardinal Health, a pharmaceutical company, was recalling eyedrops sold under the Rugby brand following three reports of burning eyes, blurry vision, and vision loss.
Generally speaking, though, experts agree that artificial tears are safe as long as users take certain safety precautions and are aware of what they’re putting in their eyes.
“Any lubricating drop that has not been recalled and is still available on pharmacy shelves should be perfectly safe,” an email from the American Academy of Ophthalmology’s spokesperson, Dr. Christopher Starr, stated.
Peak eyedrop season occurs in the autumn, when cold, dry air emerges and ragweed and other allergens proliferate, according to experts. 2020 saw at least 117 million Americans use eyedrop products, according to data research firm Statista. By 2024, the group projects that figure to have risen to over 123 million.
But the FDA issued a number of grave cautions regarding the contamination of these widely used products in 2023.
- The Centers for Disease Control and Prevention advised individuals to discontinue using EzriCare Artificial Tears in January while they looked into possible bacterial infections connected to the product. It was found in a matter of months that Pseudomonas aeruginosa, an uncommon strain of drug-resistant bacteria, had infected 81 patients. Four required surgery to remove an eyeball, four died, and fourteen became blind. The manufacturer of Delsam Pharma’s Artificial Ointment and Tears, as well as EzriCare Artificial Tears, also had two other products recalled. Since May, there have been no new reports of injuries.
- The FDA announced in August that it had found bacterial and fungal contamination in Light Eyes MSM Eye Drops-Eye Repair and Dr. Berne’s MSM Drops 5% Solution. Methyl sulfonyl methane, or MSM, was an ingredient in the products that wasn’t permitted to be used in eyedrops. No reports of injuries resulting from the drops were received.
- Following the discovery of bacteria in the facility used to manufacture 27 different types of generic eyedrops by inspectors earlier this week, the FDA issued a warning against their use. The drops were removed from store shelves after being sold at CVS, Rite Aid, Target, and Walmart, among other major retailers.
The risks associated with using the drops are a concern for experts and health officials.
The FDA stated via email, “We urge consumers to stop using these products, as it could result in an eye infection.”
The reason behind the agency’s close examination of this specific facility—which remains nameless—is unknown.
Why are the eyes so delicate?
Our immune systems are programmed to activate when bacteria enter our bodies through food or skin cuts. To form scabs, white blood cells proliferate. Attack by stomach acids.
However, eyes are especially susceptible to foreign invaders. They “don’t have that level of protection,” according to Cleveland’s Case Western Reserve University assistant professor of medicine Dr. Morgan Morelli. “You’re putting the product straight onto the eye.”
The corneal dome, the outermost layer of the eye, is devoid of blood vessels that are typically essential to the immune system’s functioning because the eye “has to maintain a crystal clarity,” according to Dr. Ronald Benner, president of the American Optometric Association.
Allergens can cause minor scratches on the surface of the eye, which can lead to bacterial infections when people rub and scratch their eyes.
“When you have an infection in that tissue, the body can’t fight it as quickly,” Benner explained. “Putting a drop in that’s contaminated makes things worse.”
Expert advice: Avoid using generic eyedrops.
Experts advise customers to check their medicine cabinets, purses, desk drawers, junk drawers, and any other locations where eyedrops might be kept as a first step in light of the recalls. The FDA has advised against using the following list of recent drops.
Additionally, experts advise sticking to name-brand eyedrops and other artificial tear products.
“There are some generics out there that are probably fine and safe, but you never know where they are manufactured,” Benner stated. “You never know how long they’ve been on the shelf.”
Despite the fact that brands are typically more expensive than generics, Morelli agreed. She mentioned that she hesitated recently when she had to pay $30 for her own eyedrops. “When it comes to the eyes, you’re probably safer spending more money and going with a brand name,” Morelli stated.
Starr, who is also an associate professor of ophthalmology at New York’s Weill Cornell Medicine, advises all patients to make sure that the eyedrop bottles have not expired.
“If expired, please discard them, as there is a higher risk of contamination even with nonrecalled, well-manufactured eyedrops,” he stated.
More professional advice:
- Hand-wash before applying eyedrops.
- Despite their cleanliness, make sure the eyedropper’s tip stays away from anything, including your hands. This covers every area of the eye, even the lashes.
- Make sure the eyedrops have not expired and are labeled as “sterile”.
- Eyedrop bottles should not be shared, not even by relatives. “We do see eye infections spread from person to person,” Benner stated. “Your tears ought to be your tears. You are not to share them with anyone else.