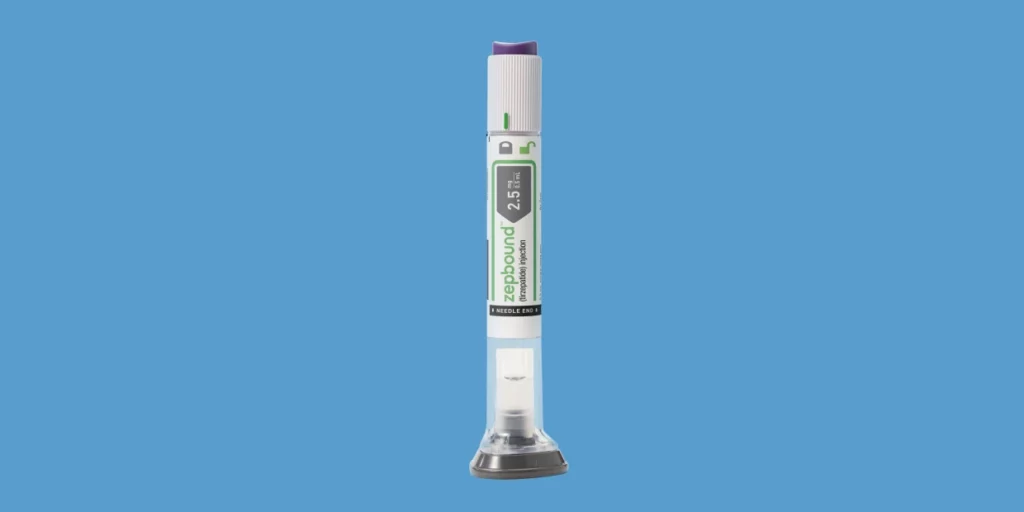
The Food and Medication Organization supported a solicitation by Eli Lilly on Wednesday to start showcasing its tirzepatide drug, which is marked as Mounjaro for diabetes, under another brand for weight reduction too.
While Mounjaro had previously been utilized by certain patients “off-name” for weight reduction, the new FDA endorsement will permit the drugmaker to start authoritatively selling and showcasing tirzepatide — marked as Zepbound — for weight reduction as well.
Zepbound will be accessible for patients in the U.S. before the year’s over, the drugmaker said.
The organization expressed Wednesday in a news discharge that the prescription, directed with an infusion pen, will be sold at a less expensive rundown value than its semaglutide rivals from Novo Nordisk, which are marked as Wegovy for weight reduction and Ozempic for diabetes.
“New treatment choices carry desire to the many individuals with stoutness who battle with this illness and are looking for better choices for weight the executives,” Joe Nadglowski, Chief of the Corpulence Activity Alliance, said in Eli Lilly’s delivery. The gathering gets subsidizing from Eli Lilly and other drug and medical organizations.
The FDA’s endorsement of Zepbound was somewhat founded on a preliminary of grown-ups without diabetes, which tracked down that members — who found the middle value of 231 pounds toward the beginning of the preliminary — who were given the most elevated supported portion lost around 18% of their body weight contrasted with fake treatment.
“Considering expanding paces of both heftiness and overweight in the US, the present endorsement addresses a neglected clinical need,” the FDA’s Dr. John Sharretts, head of the organization’s Division of Diabetes, Lipid Issues, and Weight, said in a news discharge.
While there have not been results from enormous clinical preliminaries looking at Novo Nordisk’s and Eli Lilly’s prescriptions no holds barred, there is an exploration to recommend Zepboud could outflank Ozempic.
A meta-examination introduced at the yearly gathering of the European Relationship for the Investigation of Diabetes in October closed tirzepatide was “more viable for weight reduction than semaglutide, with a bigger weight reduction impact at higher dosages,” however recognized limits in attempting to make an immediate correlations of the two.
In a report recently contrasting semaglutide and tirzepatide for diabetics, the Establishment for Clinical and Financial Survey presumed that tirzepatide showed “more noteworthy decrease” in weight and other key markers, however “had a more prominent occurrence of gastrointestinal secondary effects, extreme unfavorable occasions, and end contrasted and semaglutide.”
Zepbound conveys the gamble of a variety of possible aftereffects, the FDA says, including sickness, the runs, spewing, obstruction, and going bald.
Like with other weight reduction drugs in this class, a portion of Zepbound’s secondary effects could be serious.
Individuals with a background marked by serious gastroparesis, or stomach loss of motion, shouldn’t utilize the medication, the FDA says.
Eli Lilly and Novo Nordisk have both confronted claims that their medications can cause stomach loss of motion. The FDA as of late moved to recognize reports of ileus, or a blockage in the digestion tracts, on Ozempic’s mark.
The office likewise takes note of that others could be at higher gamble of additional serious issues from Zepbound, incorporating patients with a background marked by medullary thyroid malignant growth, pancreas irritation, or extreme gastrointestinal illness.
It likewise ought not be joined with other alleged GLP-1 receptor agonist drugs, which incorporate its kin Mounjaro, as well as Wegovy and Ozempic.
“The security and adequacy of coadministration of Zepbound with different prescriptions for weight the board have not been laid out,” the organization says.