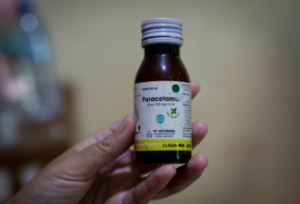
Jakarta, Indonesia – On August 23, 2024, an Indonesian court issued a landmark ruling in the case of toxic cough syrup that resulted in the deaths and injuries of over 200 children. Here are the top five key facts about this critical court decision:
1. Court-Ordered Compensation: Up to 60 Million Rupiah Per Family
The Central Jakarta court has ordered two companies—Afi Farma and CV Samudera Chemical—to pay compensation to the families affected by the toxic cough syrup. Each family whose child died or was injured will receive up to 60 million rupiah (approximately $3,850). This ruling comes after a civil lawsuit was filed by 25 families demanding justice for their children.
2. Tragic Impact: Over 200 Children Affected
The toxic cough syrup tragedy emerged in 2022 when children across Indonesia began falling seriously ill after consuming the syrup. The poisoning led to acute kidney disease, killing over 200 children. Approximately 120 children survived, but many are now living with severe disabilities.
3. High Levels of Toxic Chemicals: Ethylene Glycol Found
Investigations revealed that the cough syrups contained alarmingly high levels of ethylene glycol (EG), a chemical typically used in antifreeze and brake fluid. The EG concentration in the syrups was found to be as high as 99 percent, far exceeding the international safety limit of 0.1 percent. This toxic level of EG was directly linked to the severe health issues experienced by the children.
4. Legal Actions: Multiple Cases and Rulings
The toxic syrup scandal led to both civil and criminal legal actions. In the recent civil case, the court found Afi Farma and CV Samudera Chemical at fault, ordering them to pay the affected families. Earlier, in a criminal trial, Afi Farma was convicted of negligence for failing to test the ingredients provided by CV Samudera Chemical, resulting in the distribution of the deadly syrup.
5. Ongoing Consequences: Calls for Stricter Regulations
The ruling has prompted renewed calls for tighter regulatory oversight in Indonesia’s pharmaceutical industry. Despite the compensation, many believe the amount is insufficient to account for the deep emotional and physical toll on the affected families. The case has also led to increased scrutiny of the country’s drug safety standards and the need for more rigorous testing protocols.

The Central Jakarta court’s decision represents a significant step in addressing the devastating consequences of the toxic cough syrup case. While the compensation offers some relief to the families, the ruling underscores the critical need for improved safety measures in the pharmaceutical sector to prevent such tragedies from happening again.
.An Indonesian court has ordered two companies, Afi Farma and CV Samudera Chemical, to pay compensation to the families of children who died or were seriously injured after consuming toxic cough syrup. The ruling mandates payments of up to 60 million rupiah (approximately $3,850) per affected family. The case centers around the distribution of cough syrup contaminated with ethylene glycol (EG), a toxic chemical commonly found in industrial products like antifreeze. Over 200 children in Indonesia died from acute kidney injury after consuming the syrup, while around 120 others survived, some with severe disabilities.
The court’s decision comes after a civil lawsuit was filed by families against Indonesia’s food and drugs agency (BPOM), the Health Ministry, and several pharmaceutical companies. While Afi Farma and CV Samudera were found liable, the BPOM and the Health Ministry were cleared of any wrongdoing.
This incident is part of a larger pattern of similar cases, with toxic cough syrups causing child fatalities in other countries, including Gambia and Uzbekistan. The pharmaceutical companies involved have expressed dissatisfaction with the court’s ruling and are considering further legal action.
The outcome has highlighted issues related to pharmaceutical oversight and regulation in Indonesia, raising concerns about the safety standards for drug production and distribution in the country.